Key Takeaways
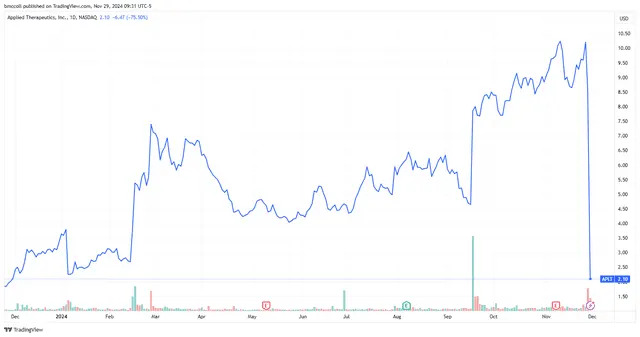
Applied Therapeutics ( APLT ) shares cratered Friday as the biopharmaceutical firm reported that the Food and Drug Administration (FDA) rejected its application for its experimental treatment for a rare metabolic disease known as Galactosemia.
The company said it had received a Complete Response Letter (CRL) from the FDA that explained that after it completed a review of the drug, govorestat, it was unable to approve Applied Therapeutics' New Drug Application (NDA) in its current form. The FDA pointed to "deficiencies in the clinical application."
Patients with Galactosemia cannot process the simple sugar galactose. The company noted that approximately 3,300 people in the U.S. suffer from the disease, as well as 4,400 in the European Union (EU) . "Newborn screening for Galactosemia is mandatory in the U.S. and most E.U. countries," the company said.
Founder and Chief Executive Officer (CEO) Shoshana Shendelman expressed disappointment in the decision, arguing that govorestat "has the potential to change the lives of patients with Galactosemia, which we believe is evidenced by the breadth of efficacy and safety data demonstrating its ability to stop the decline on progressive clinical outcomes, including cognition and behavior."
Applied Therapeutics Plans To Request Meeting or Appeal FDA Decision
Applied Therapeutics added that it is "reviewing the feedback from the FDA and plans to immediately request a meeting to discuss requirements for a potential resubmission of the NDA or appeal of the decision along with appropriate next steps."
Shares of Applied Therapeutics lost about three-quarters of their value at the start of morning trading.